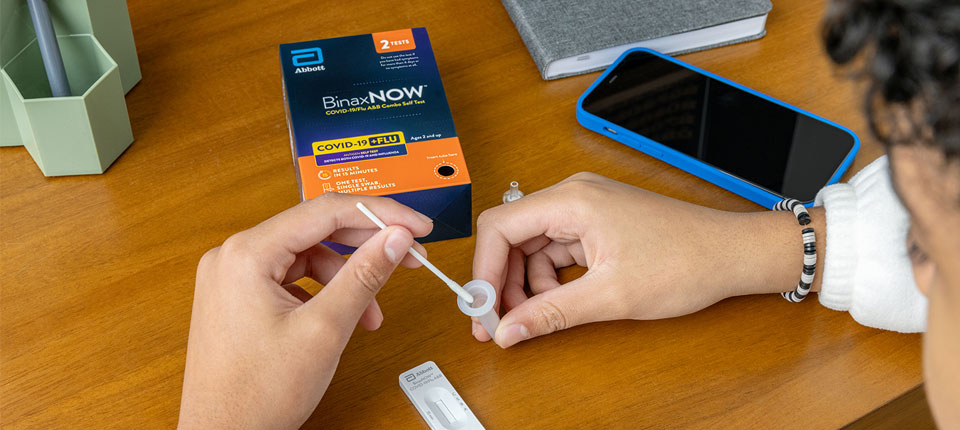
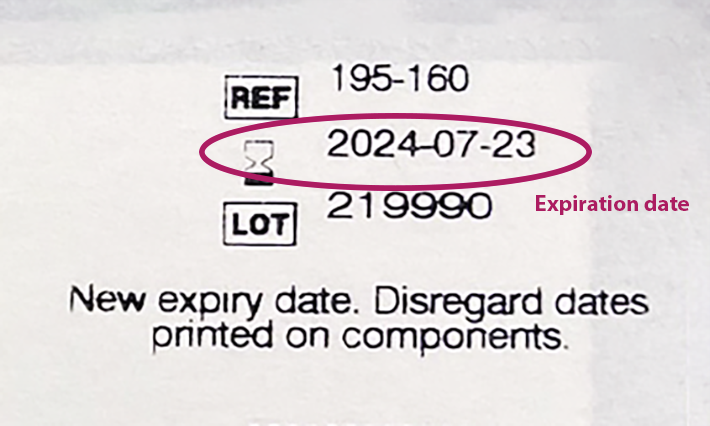
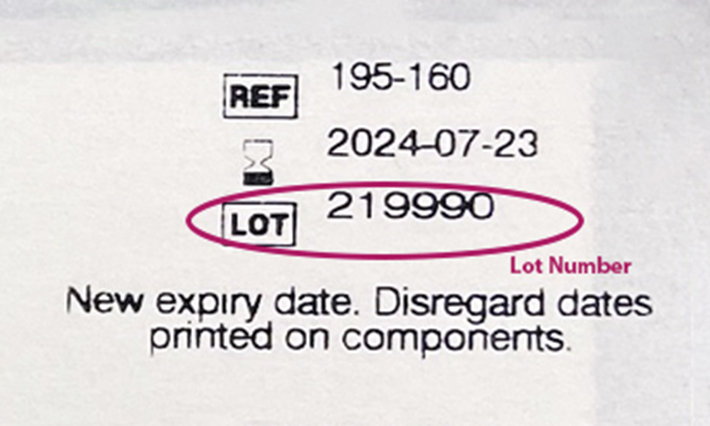
3. Search the lot number to find the expiration date extension. Input your lot number via our Expiration Date Look Up Tool at rapidtest.abbott/binaxnow to confirm the expiration date extension of your test.
Reminders:
- Finding the lot number is most important. If your test’s lot number appears, trust the extended expiration date, even if the printed expiration date doesn’t match.
- If your test’s lot number doesn’t appear, the test’s expiration date may not have been extended. You can recycle the box, but should dispose of the test card, nasal swab and test solution in common household waste, in line with the test’s instructions for use.
Where can I find tests?
Rapid tests are a handy tool as we keep dealing with COVID-19 — especially as we see a rise in cases as we plan gatherings with friends and loved ones.
Because of this, we continue to ensure BinaxNOW COVID-19 Self Tests are available when and where you need them. You can find them online and at your local retailers, including Amazon, CVS, RiteAid, Sam’s Club, Walgreens and Walmart.
How is Abbott tracking new COVID variants?
Through the Abbott Pandemic Defense Coalition, we have a network of research, academic and public health collaborators strategically placed around the world that are actively sequencing viruses to look for the next viral threat, including new COVID-19 variants.
Having this established network gives us a way to quickly share new information and verify our tests. This type of global collaboration is critical to fighting variants and preventing the next pandemic.
COVID testing is crucial to tackling respiratory season. It helps keep you and your family prepared for everything this fall has in store.
This story was originally published on October 23, 2023, and updated on August 27, 2024.