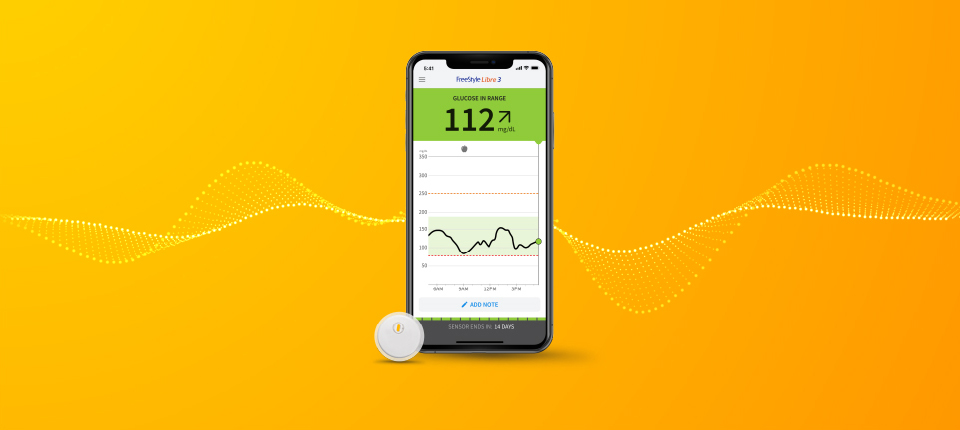
The FreeStyle Libre 2 Flash Glucose Monitoring System is a continuous glucose monitoring (CGM) device with real time alarms capability indicated for the management of diabetes in persons age 4 and older. It is intended to replace blood glucose testing for diabetes treatment decisions, unless otherwise indicated.
The System also detects trends and tracks patterns and aids in the detection of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments. Interpretation of the System readings should be based on the glucose trends and several sequential readings over time.
The System is also intended to autonomously communicate with digitally connected devices. The System can be used alone or in conjunction with these digitally connected devices where the user manually controls actions for therapy decisions.
CONTRAINDICATIONS
Automated Insulin Dosing: The System must not be used with automated insulin dosing (AID) systems, including closed loop and insulin suspend systems.
MRI/CT/Diathermy: The System must be removed prior to Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or high-frequency electrical heat (diathermy) treatment. The effect of MRI, CT scans, or diathermy on the performance of the System has not been evaluated. The exposure may damage the Sensor and may impact proper function of the device which could cause incorrect readings.
WARNINGS
Before you use the FreeStyle Libre 2 System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide and Interactive Tutorial give you quick access to important aspects and limitations of the System. The User’s Manual includes all safety information and instructions for use. Talk to your health care professional about how you should use your Sensor glucose information to help manage your diabetes.
Failure to use the System according to the instructions for use may result in you missing a severe low blood glucose or high blood glucose event and/or making a treatment decision that may result in injury. If your glucose alarms and readings from the System do not match symptoms or expectations, use a fingerstick blood glucose value from a blood glucose meter to make diabetes treatment decisions. Seek medical attention when appropriate.
Do not ignore symptoms that may be due to low or high blood glucose: If you are experiencing symptoms that are not consistent with your glucose readings, consult your health care professional.
Use your blood glucose meter to make diabetes treatment decisions when you see the Check Blood Glucose symbol during the first 12 hours of wearing a Sensor, if your Sensor glucose reading does not match how you feel, or if the reading does not include a number.
Choking hazard: The System contains small parts that may be dangerous if swallowed.
CAUTIONS AND LIMITATIONS
Below are important cautions and limitations to keep in mind so you can use the System safely. They are grouped into categories for easy reference.
What to know about Glucose Alarms:
For you to receive alarms, they must be on and your Reader should be within 20 feet of you at all times. The transmission range is 20 feet unobstructed. If you are out of range, you may not receive glucose alarms.
To prevent missed alarms, make sure the Reader has sufficient charge and that sound and/or vibration are turned on.
Alarms you receive do not include your glucose reading so you must scan your Sensor to check your glucose.
What to know before using the System:
Review all product information before use.
Take standard precautions for transmission of blood borne pathogens to avoid contamination.
Make sure that your Reader and Sensor kits are kept in a safe place, under your control. This is important to help prevent anyone from accessing or tampering with the System.
Who should not use the System:
Do not use the System in people less than 4 years of age. The System is not cleared for use in people under 4 years of age.
Do not use the System if you are pregnant, on dialysis, or critically ill. The System is not cleared for use in these groups and it is not known how different conditions or medications common to these populations may affect performance of the System.
Performance of the System when used with other implanted medical devices, such as pacemakers, has not been evaluated.
What should you know about wearing a Sensor:
Wash application site on the back of your upper arm using a plain soap, dry, and then clean with an alcohol wipe. This will help remove any oily residue that may prevent the Sensor from sticking properly. Allow site to air dry before proceeding. Carefully preparing the site according to these instructions will help the Sensor stay on your body for the full 14 day wear period and help prevent it from falling off early.
The Sensor can be worn for up to 14 days. Remember to always have your next Sensor available before your current one ends so you can keep getting your glucose readings.
You must scan the Sensor to get your real-time current glucose level as the Reader will not provide this information without a scan.
In the event that your Sensor stops working and you do not have another Sensor readily available, you must use an alternate method to measure your glucose levels and inform your treatment decisions.
The System is designed to detect certain conditions which may occur where the Sensor is not working as intended and shut it off, telling you to replace your Sensor. This may occur if the Sensor gets knocked off from the skin or if the System detects that the Sensor may not be performing as intended. Contact Customer Service if you receive a Replace Sensor message before the end of the 14 day wear period. Customer Service is available at 1-855-632-8658 7 Days a Week from 8AM to 8PM Eastern Standard Time.
Some individuals may be sensitive to the adhesive that keeps the Sensor attached to the skin. If you notice significant skin irritation around or under your Sensor, remove the Sensor and stop using the System. Contact your health care professional before continuing to use the System.
Intense exercise may cause your Sensor to loosen due to sweat or movement of the Sensor. If the Sensor is becoming loose or if the Sensor tip is coming out of your skin, you may get no readings or unreliable low readings. Remove and replace your Sensor if it starts to loosen and follow the instructions to select an appropriate application site. Do not attempt to reinsert the Sensor. Contact Customer Service if your Sensor becomes loose or falls off before the end of the wear period. Customer Service is available at 1-855-632-8658 7 Days a Week from 8AM to 8PM Eastern Standard Time.
Do not reuse Sensors. The Sensor and Sensor Applicator are designed for single use. Reuse may result in no glucose readings and infection. Not suitable for re-sterilization. Further exposure to irradiation may cause unreliable low results.
If a Sensor breaks inside your body, call your health care professional.
How to Store the Sensor Kit:
Store the Sensor Kit between 36°F and 82°F. Storage outside of this range may cause inaccurate Sensor glucose readings.
If you suspect that the temperature may exceed 82°F (for example, in an un-airconditioned home in summer), you should refrigerate your Sensor Kit. Do not freeze your Sensor Kit.
Store your Sensor Kit in a cool, dry place. Do not store your Sensor Kit in a parked car on a hot day.
Store the Sensor Kit between 10-90% non-condensing humidity.
When not to use the System:
Do NOT use if the Sensor Kit package, Sensor Pack, or Sensor Applicator appear to be damaged or already opened due to risk of no results and/or infection.
Do NOT use if Sensor Kit contents are past expiration date.
Do NOT use if the Reader appears to be damaged due to risk of electric shock and/or no results.
What to know before you Apply the Sensor:
The Sensor Pack and Sensor Applicator are packaged as a set (separately from the Reader) and have the same Sensor code. Check that the Sensor codes match before using your Sensor Pack and Sensor Applicator. Do not use Sensor Packs and Sensor Applicators with different Sensor codes together as this will result in incorrect glucose readings.
Wash application site on the back of your upper arm using a plain soap, dry, and then clean with an alcohol wipe. This will help remove any oily residue that may prevent the Sensor from sticking properly.
Allow site to air dry before proceeding. Carefully preparing the site according to these instructions will help the Sensor stay on your body for the full 14 day wear period and help prevent it from falling off early.
Clean hands prior to Sensor handling/insertion to help prevent infection.
Change the application site for the next Sensor application to prevent discomfort or skin irritation.
Only apply the Sensor to the back of the upper arm. If placed in other areas, the Sensor may not function properly.
Select an appropriate Sensor site to help the Sensor stay attached to the body and prevent discomfort or skin irritation. Avoid areas with scars, moles, stretch marks, or lumps. Select an area of skin that generally stays flat during normal daily activities (no bending or folding). Choose a site that is at least 1 inch away from an insulin injection site.
When is Sensor Glucose different from Blood Glucose:
Physiological differences between the interstitial fluid and capillary blood may result in differences in glucose readings between the System and results from a fingerstick test using a blood glucose meter.
Differences in glucose readings between interstitial fluid and capillary blood may be observed during times of rapid change in blood glucose, such as after eating, dosing insulin, or exercising.
What to know about X-Rays:
The Sensor should be removed prior to exposing it to an X-ray machine. The effect of X-rays on the performance of the System has not been evaluated. The exposure may damage the Sensor and may impact proper function of the device to detect trends and track patterns in glucose values during the wear period.
When to remove the Sensor:
If the Sensor is becoming loose or if the Sensor tip is coming out of your skin, you may get no readings or unreliable readings, which may not match how you feel. Check to make sure your Sensor has not come loose. If it has come loose, remove it, apply a new one, and contact Customer Service.
If you believe your glucose readings are not correct or are inconsistent with how you feel, perform a blood glucose test on your finger to confirm your glucose. If the problem continues, remove the current Sensor, apply a new one, and contact Customer Service. Customer Service is available at 1-855-632-8658 7 Days a Week from 8AM to 8PM Eastern Standard Time.
What to know about the Reader’s Built-in Meter:
The FreeStyle Libre 2 Reader has a built-in blood glucose meter that is designed to be used only with FreeStyle Precision Neo blood glucose test strips and MediSense Glucose and Ketone Control Solution. Using other test strips with the Reader’s built-in meter will produce an error or cause the Reader’s built-in meter to not turn on or start a test. The Reader’s built-in meter does not have





FOLLOW ABBOTT