Abbott Launches COVID-19 Antibody Test
New test can help determine if someone was infected with the virus and if the person has developed antibodies.
Apr. 15, 2020
- Copy Link
- Share on X
- Share on Facebook
- Share on Linkedin
Abbott has launched its third test for coronavirus (COVID-19) and is shipping tests to hospitals across the U.S.
The test is a serology test – also called an antibody test – which could be a critical next step in battling this virus.
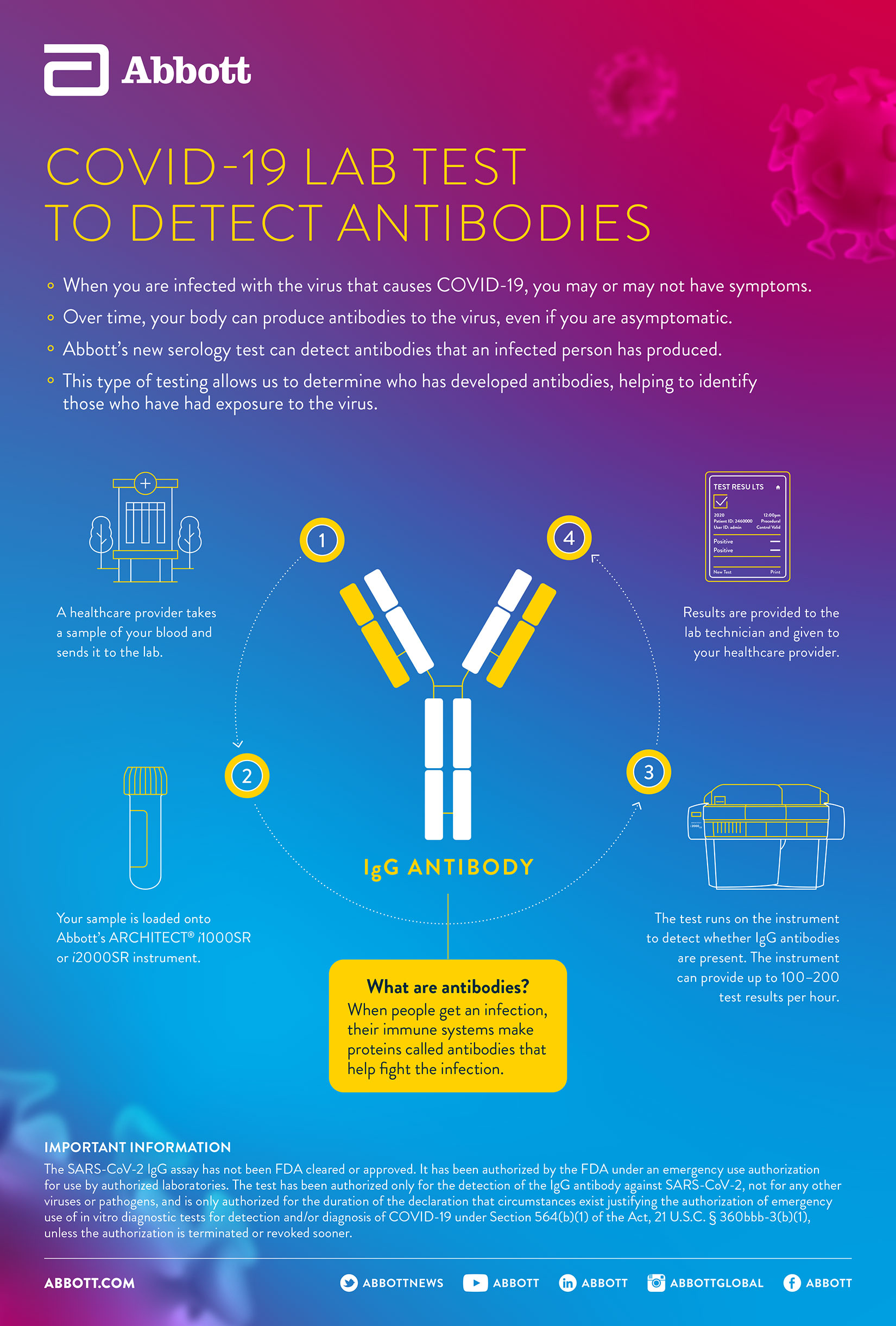
Abbott's test helps to detect the IgG antibody to SARS-CoV-2. An antibody is a protein that the body produces in the late stages of infection and may remain for up to months and possibly years after a person has recovered. Detecting these IgG antibodies will help determine if a person was previously infected with the virus that causes COVID-19.
The new antibody test is to be used on Abbott's ARCHITECT i1000SR and i2000SR laboratory instruments, which can run up to 100-200 tests an hour.1
Abbott first made the test available as part of the U.S. Food and Drug Administration's notification without an Emergency Use Authorization (EUA) pathway that was outlined for COVID-19 diagnostic tests during the public health emergency. Since then, we've received Emergency Use Authorization (EUA) from the FDA and CE Mark in Europe.
We're significantly scaling up our manufacturing for antibody testing and expect to ship close to 1 million tests to U.S. customers this week and 4 million of the antibody tests during April.
And that's just the start.
Abbott plans to ship 20 million antibody tests in the U.S. in June and beyond as we expand our testing capabilities to our Alinity i lab system.
This antibody test adds to Abbott’s existing COVID-19 molecular tests that are already being used – our m2000 lab test and our rapid, ID NOW point-of-care test.
Antibody tests, the next step in the COVID-19 battle
While molecular testing (such as Abbott’s m2000 and point-of-care tests) identify people with the virus, antibody tests can tell whether someone has been previously infected.
This type of knowledge will enable scientists to better understand how long these antibodies stay in the body and if they provide immunity. This information can also help public health officials understand how widespread the outbreak is and could help support the development of treatments and vaccines for COVID-19.
Although Abbott’s antibody test is new, the two ARCHITECT instruments it runs on are already being used for critical diagnostic tests in labs worldwide. More than 2,000 of the instruments are used in the U.S.
"Antibody testing has the potential to unlock a lot of unknowns about this novel virus," said John Hackett, divisional vice president of Applied Research and Technology, Diagnostics, Abbott. "Having tests that can work in different healthcare settings is critical to our understanding of the virus and to helping give healthcare providers answers they need about their patients."
Learn more about how Abbott is helping frontline workers tackle the COVID-19 pandemic.
This story was originally published on April 15, 2020. It was updated on April 27, 2020 to include reference that our antibody test received U.S. emergency use authorization and that we received CE Mark.
Reference
1All ARCHITECT analyzers are Class 1 laser products.
For the latest on Abbott’s life-changing technology, get updates directly in your inbox.