Important Safety Information
By Prescription Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
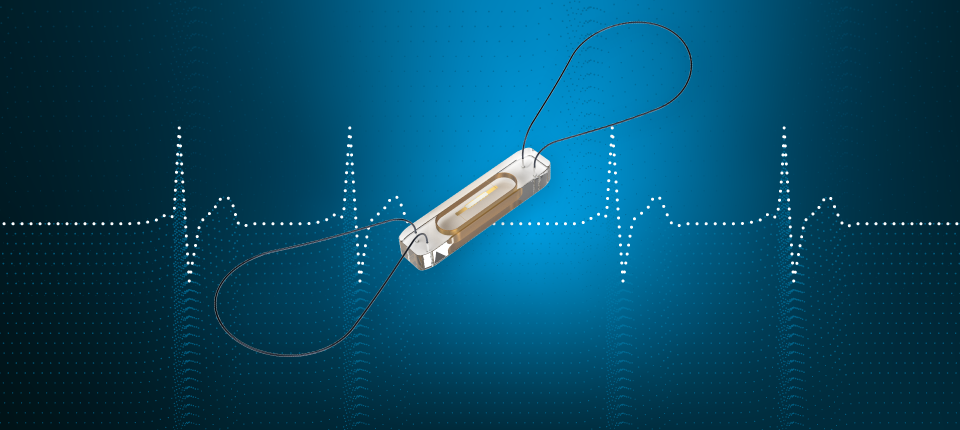
CardioMEMS™ HF System Indications and Usage: The CardioMEMS HF System is indicated for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in NYHA Class II or III heart failure patients who either have been hospitalized for heart failure in the previous year and/or have elevated natriuretic peptides. The hemodynamic data are used by physicians for heart failure management with the goal of controlling pulmonary artery pressures and reducing heart failure hospitalizations.
Contraindications: The CardioMEMS™ HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant.
CardioMEMS™ HF System Potential Adverse Events: Potential adverse events associated with the implantation procedure include, but are not limited to, the following: air embolism, allergic reaction, infection, delayed wound healing, arrhythmias, bleeding, hemoptysis, hematoma, nausea, cerebrovascular accident, thrombus, cardiovascular injury, myocardial infarction, death, embolization, thermal burn, cardiac perforation, pneumothorax, thoracic duct injury and hemothorax.
myCardioMEMS™ Mobile App Limitations: Patients must use their own Apple‡ or Android‡ mobile device to receive and transmit information to the myCardioMEMS™ Mobile App. To do so the device must be powered on, app must be installed and data coverage (cellular or Wi-Fi‡) available. The myCardioMEMS™ App can provide notification of medication adjustments and reminders, requests for lab work and acknowledgment that the PA pressure readings have been received. However there are many internal and external factors that can hinder, delay, or prevent acquisition and delivery of the notifications and patient information as intended by the clinician. These factors include: patient environment, data services, mobile device operating system and settings, clinic environment, schedule/configuration changes, or data processing.
GALLANT CRT-D
Rx Only
Intended Use: The Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy Defibrillator (CRT-D) devices are intended to provide ventricular antitachycardia pacing and ventricular cardioversion/defibrillation. The CRT-D devices are also intended to resynchronize the right and left ventricles.
The myMerlinPulse™ mobile application is intended for use by people who have an Abbott Medical implanted heart device and access to a mobile device. The app provides remote monitoring capability of the implanted heart device by transmitting information from the patient’s implanted heart device to the patient’s healthcare provider.
Indications: The ICD and CRT-D devices are indicated for automated treatment of life-threatening ventricular arrhythmias. CRT-D devices are also indicated to treat symptoms in patients who have congestive heart failure with ventricular dyssynchrony.
In addition, dual chamber ICD and CRT-D devices with the AT/AF detection algorithm are indicated in patients with atrial tachyarrhythmias or those patients who are at significant risk of developing atrial tachyarrhythmias.
MR Conditional ICDs and CRT-Ds are conditionally safe for use in the MRI environment when used in a complete MR Conditional system and according to instructions in the MRI-Ready Systems manual. Scanning under different conditions may result in severe patient injury, death or device malfunction.
The myMerlinPulse™ mobile application is indicated for use by patients with supported Abbott Medical implanted heart devices.
Contraindications: Contraindications for use of the pulse generator system include ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction.
The myMerlinPulse™ mobile application is contraindicated for use with any implanted medical device other than supported Abbott Medical implanted heart devices.
Adverse Events: Possible adverse events associated with the implantation of the pulse generator system include the following: Arrhythmia (for example, accelerated or induced), Bradycardia, Cardiac or venous perforation, Cardiac tamponade, Cardiogenic shock, Death, Discomfort, Embolism, Endocarditis, Erosion, Exacerbation of heart failure, Excessive fibrotic tissue growth, Extracardiac stimulation (phrenic nerve, diaphragm, pectoral muscle), Extrusion, Fluid accumulation within the device pocket, Formation of hematomas, cysts, or seromas, Heart block, Hemorrhage, Hemothorax, Hypersensitivity, including local tissue reaction or allergic reaction, Infection,Keloid formation, Myocardial damage, Nerve damage, Occlusion/Thrombus, Pericardial effusion, Pericarditis, Pneumothorax, Pulmonary edema, Syncope, Thrombosis, Valve damage. Complications reported with direct subclavian venipuncture include pneumothorax, hemothorax, laceration of the subclavian artery, arteriovenous fistula, neural damage, thoracic duct injury, cannulation of other vessels, massive hemorrhage and rarely, death. Among the psychological effects of device implantation are imagined pulsing, depression, dependency, fear of premature battery depletion, device malfunction, inappropriate pulsing, shocking while conscious, or losing pulse capability. Possible adverse device effects include complications due to the following: , Abnormal battery depletion, Conductor fracture, Device-programmer communication failure, Elevated or rise in defibrillation/cardioversion threshold, Inability to defibrillate or pace, Inability to interrogate or program due to programmer or device malfunction, Incomplete lead connection with pulse generator, Inhibited therapy including defibrillation and pacing, Inappropriate therapy (for example, shocks and antitachycardia pacing [ATP] where applicable, pacing), Interruption of function due to electrical or magnetic interference, Intolerance to high rate pacing (for example dyspnea or discomfort), Lead abrasion, Lead fracture, Lead insulation damage, Lead migration or lead dislodgement, Loss of device functionality due to component failure, Pulse generator migration, Rise in DFT threshold, Rise in pacing threshold and exit block, Shunting of energy from defibrillation paddles, System failure due to ionizing radiation. Additionally, potential adverse events associated with the implantation of a coronary venous lead system include the following: Allergic reaction to contrast media, Breakage or failure of implant instruments, Prolonged exposure to fluoroscopic radiation, Renal failure from contrast media used to visualize coronary veins. Refer to the User’s Manual for detailed intended use, indications, contraindications, warnings, precautions and potential adverse events.
No potential adverse events have been identified with use of the myMerlinPulse™ mobile application.
† Competition defined as LV Only and BiV Simultaneous pacing modes. Modes 1 and IV of the referenced JAHA data.
‡ Fixed-tilt group of patients with competitive devices only achieved 83% success for maintaining a 10J safety margin
References:
1. Varma N, O’Donnell D, Bassiouny M, et al. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7:e007489.http://jaha.ahajournals.org/content/7/3/e007489. Accessed April 17, 2018.
2. Wisnoskey BJ, Cranke G, Cantillon DJ, and Varma N. “Feasibility of Device-Based Electrical Optimization via Application of the Negative AV Hysteresis Algorithm during Cardiac Resynchronization Therapy (CRT).” Heart Rhythm. 2016; 13 (5S): S443.
3. Varma, N., Hu, Y., Connolly, A. T., Thibault, B., Singh, B., Mont, L., … & Zareba, W. (2021). Gain in realworld cardiac resynchronization therapy efficacy with SyncAV dynamic optimization: Heart failure hospitalizations and costs. Heart Rhythm.
4. Jastrzebski M, Baranchuk A, Fijorek K, Kisiel R, Kukla P, Sondej T, Czarnecka D. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. Europace. 2019 Feb 1;21(2):281-289. doi: 10.1093/europace/euy254. PMID: 30403774.
5. Based on over 560,000 episodes (20,000 patients). Performance of VF Therapy Assurance Feature. Abbott Clinical Summary.
6. Data on file. 60101422 Internal Validation Report. Total 2019 global high-voltage implants, all manufacturers, estimated to be 440,434 units (Source: Abbott Market Research).
7. Roland X. Stroobandt MD, PhD; Mattias F Duytschaever MD, PhD; Terest Strisciuglio MD; Frederic E.Van Heuverswyn MD; Liesbeth Timmers MD; Jan De Pooter MD, PhD; Sebastien Knecht MD, PhD; Yves R. Vanderkerckhove MD; Andreas Kucher PhD; Rene H. Tavernier MD, PhD. Failure to detect life-threatening arrhythmias in ICDs using single-chamber detection criteria. Pacing Clin Electrophysiology.2019;42:583-594. DOI:10.1111/pace.13610.3.
8. Gabriels J, Budzikowski AS, Kassotis JT. Defibrillation waveform duration adjustment increases the proportion of acceptable defibrillation thresholds in patients implanted with single-coil defibrillation leads. Cardiology. 2013;124(2):71.
ENSITE X
EnSite™ X EP System ISI
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available) or at manuals.sjm.com or eifu.abbottvascular.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events.
Indications: The EnSite X EP System is a suggested diagnostic tool in patients for whom electrophysiology studies have been indicated.
The EnSite X EP System provides information about the electrical activity of the heart and displays catheter location during conventional electrophysiological (EP) procedures.
Warnings: For patient safety, any connections that directly connect the patient to the EnSite X EP System must be routed through the appropriate modules: EnSite X EP System SurfaceLink Module, EnSite X EP System 20 pin Catheter Input Module, EnSite X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite X EP System Amplifier.
When using the EnSite X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables.
The use of this device in conjunction with radio frequency ablation, as a part of the diagnosis and treatment of cardiac arrhythmias, may pose an increased risk of adverse events such as cardiac perforation, myocardial infarction, air embolism, and hematoma requiring surgical repair and/or blood transfusion.
Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals.
Only connect items that have been specified as part of the EnSite X EP System or compatible with the EnSite X EP System to the multiple socket-outlets.
The EnSite X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location.
The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only.
Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift.
Precautions: Ensure that surface electrodes, Patient Reference Sensors, and associated connectors do not contact one another, electrical ground, or metallic objects.
EnSite X EP System components should be connected to power through an isolation transformer or the multiple socket outlet supplied with the system carts. Connecting equipment directly to a wall outlet may result in excessive leakage current.
Do not operate the EnSite X EP System Field Frame within 10 m of another operating Field Frame.
Do not place the EnSite X EP System Field Frame Cable inside the measurement volume or wrap it around the EnSite X EP System Field Frame, as it may create a magnetic interference.
Metallic equipment used in close proximity to the magnetic field during the procedure, such as a sterile drape holder, may cause metal distortion.
Do not place tool cables within 30 mm of the EnSite X EP System Field Frame Cable. If placed this close-particularly if the cables are parallel to each other the tool cable may become subject to electromagnetic interference.
Do not use the EnSite X EP System in the presence of other magnetic fields.
Do not drop the EnSite X EP System Field Frame or subject it to impact. Physical damage to the EnSite X EP System Field Frame may alter the EnSite X EP System Field Frame's factory calibration.
Related articles
-
CardioMEMS Set for Broader Use
FDA approves expanded indication, boosting access to Abbott's connected tech for earlier-stage heart failure.
-
"Life is Good": How Iris found balance with CardioMEMS
After Iris Welch's heart failure diagnosis, CardioMEMS helped her get back to traveling, cooking and family.