Test. Test. Test. It's key to beating the coronavirus pandemic, according to the World Health Organization.
That's why Abbott is leveraging our diagnostics leadership and developing more tests on more platforms, to help test millions of people around the world for COVID-19.
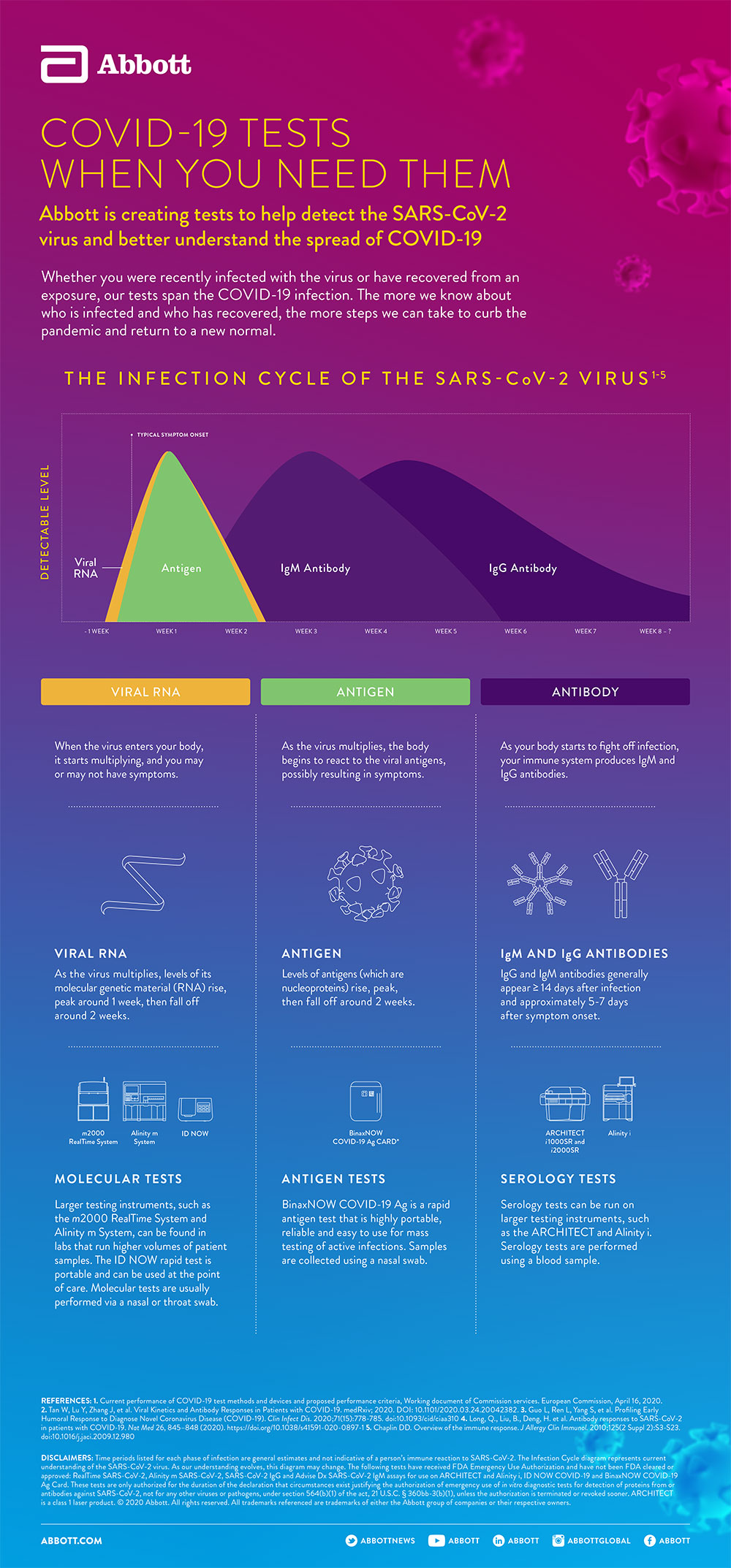
Abbott has currently developed eight COVID-19 tests that have all received Emergency Use Authorization from the U.S. Food and Drug Administration.
Among our test types — antigen, molecular and serology — there are rapid tests that can be performed at the point of care (think urgent care clinics and doctors' offices) and at home with a virtually guided service and tests that run on large, high-volume lab instruments found in hospitals and academic centers, which assess hundreds of samples at a time.
Each of our tests plays a role in helping frontline workers diagnose COVID-19 and better understand it.
Take a Closer Look
To help frontline workers identify people infected with COVID-19, Abbott launched it's lab-based molecular tests, which are used on our m2000 RealTime system (available in hospitals and molecular labs around the world) and our new Alinity m system. In a 24-hour period, the Alinity m system can run up to 1,080 tests and our m2000 system up to 470 tests.
For situations where a fast portable test is needed, Abbott developed a molecular point-of-care test that runs on our ID NOW system, which is the size of a small toaster. The test delivers results in 13 minutes or less.
The molecular tests use an upper respiratory tract swab to collect a mucus sample.
As the virus begins multiplying, antigens (proteins on the outside of the virus) can be detected. Abbott's BinaxNOW™ COVID-19 Ag Card test can identify these antigens, which are typically detected after symptoms start. Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19. The tests can be used in point-of-care settings and at home with an online service provided by eMed. The BinaxNOW-19 Ag Card is highly portable, easy-to-use and affordable, making it a valuable tool for helping reduce disease spread.
An important step in understanding COVID-19 is serology tests, also called antibody tests, which can help tell if someone has been previously infected. This type of knowledge will enable scientists to better understand how long these antibodies stay in the body and if they provide immunity. This information can also help public health officials understand how widespread the outbreak is and could help support the development of treatments and vaccines for COVID-19.
Abbott developed lab-based serology tests that detect the IgG and IgM antibodies to SARS-CoV-2 in the blood and run on our Alinity i system as well as on our ARCHITECT i1000SR and i2000SR instruments. Both Alinity i and our ARCHITECT instruments can run up to 100-200 tests an hour.1
"Testing across different platforms and settings is extremely helpful in our fight against COVID-19 to keep people healthy as we continue to flatten the curve and look for new ways to safely reopen the country," said Dr. Robert Hart, Executive Vice President and Chief Medical Officer, Ochsner Health, Louisiana.
"Molecular testing has been valuable in determining who has the active infection and how to help stop the virus from spreading," Dr. Hart said. "The new serology tests can help public health officials and policymakers make critical decisions based on how prevalent the virus is in their community."
Reference
1All ARCHITECT analyzers are Class 1 laser products.
This story was originally published on June 22, 2020. It was updated on August 26 to include reference to our announcement on Abbott’s launch of its sixth COVID-19 test, a rapid antigen test, and a companion mobile app. It was further updated on November 11 to include references that Abbott received U.S. FDA emergency use authorization and CE Mark for its seventh COVID-19 test, an antibody IgM blood test. It was further updated to include reference that Abbott’s BinaxNOW COVID-19 rapid test received U.S. FDA emergency use authorization for guided at-home use. It was again updated on June 03, 2022, and March 28, 2024.

